| Synonyms |
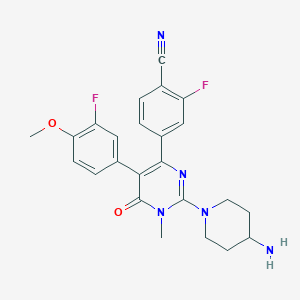
UNII-W6F4FRQ5QC; W6F4FRQ5QC; CC90011; 1821307-10-1; CC-90011 besylate; 4-[2-(4-aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl]-2-fluorobenzonitrile; Pulrodemstat; 4-(2-(4-Aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; Pulrodemstat [INN]; CC-90011 Free base; SCHEMBL17222702; GTPL11284; US10023543, Example 7; BDBM283216; US10023543, Example 85; US10023543, Example 86; NSC822744; NSC-822744; compound 11 [PMID: 33034194]; Q67009340; NC1CCN(CC1)C=1N(C(C(=C(N1)C1=CC(=C(C#N)C=C1)F)C1=CC(=C(C=C1)OC)F)=O)C; 4-(2-(4-Amino-piperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; 4-[2-(4-amino-piperidin-1-yl)-5- (3-fluoro-4-methoxy-phenyl)-1- methyl-6-oxo-1,6-dihydro- pyrimidin-4-yl]-2-fluoro- benzonitrile; Benzonitrile, 4-(2-(4-amino-1-piperidinyl)-5-(3-fluoro-4-methoxyphenyl)-1,6-dihydro-1-methyl-6-oxo-4-pyrimidinyl)-2-fluoro-; V0Y
|