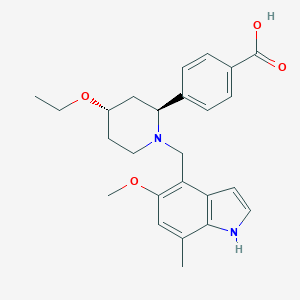
| Synonyms |
LNP-023; UNII-8E05T07Z6W; 8E05T07Z6W; 1644670-37-0; 4-((2S,4S)-4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl)benzoic acid; 4-[(2~{S},4~{S})-4-ethoxy-1-[(5-methoxy-7-methyl-1~{H}-indol-4-yl)methyl]piperidin-2-yl]benzoic acid; 4-[(2S,4S)-4-ethoxy-1-[(5-methoxy-7-methyl-1H-indol-4-yl)methyl]piperidin-2-yl]benzoic acid; Iptacopan [INN]; CHEMBL4594448; SCHEMBL16400416; GTPL10710; US9682968, Example-26a; BDBM160475; ZINC223246892; compound 41 [PMID: 32073845]; HY-127105; CS-0093107; 4-((2S,4S)-(4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl))benzoic acid; Benzoic acid, 4-((2S,4S)-4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)-2-piperidinyl)-; JGQ
|