Details of the Drug Reposition
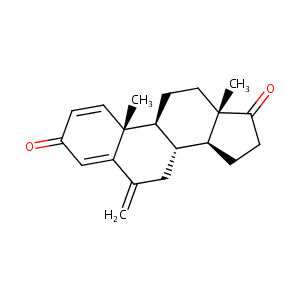
General Information of This Drug (ID: DM9HPW3)
Information on Drug Reposition of This Drug
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 Approved Indication(s)
|
|
|||||||||||||||||||||||||
|
1 Investigative Indication(s)
|
|
|||||||||||||||||||||||||