Details of the Drug Reposition
General Information of This Drug (ID: DMG1LVA)
| Drug Name | ||||||
|---|---|---|---|---|---|---|
| Synonyms | CSF-1R inhibitor (bone cancer); CSF-1R inhibitor (bone cancer), Novartis | |||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
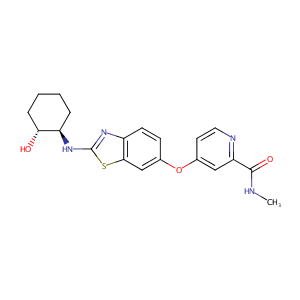 |
|||||
| 3D MOL | 2D MOL | |||||
Information on Drug Reposition of This Drug
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 Phase 2 Indication(s)
|
|
|||||||||||||||||||||||||
|
1 Phase 1 Indication(s)
|
|
|||||||||||||||||||||||||
