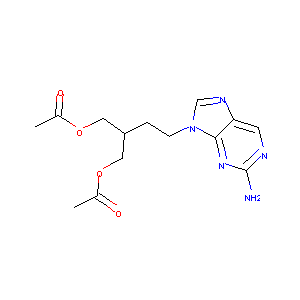
| Synonyms |
FCV; Famciclovirum; Famvir; Oravir; BRL 42810; IN1338; Anti-Farnesyl Rabbit pAb; BRL-42810; Famciclovirum [INN-Latin]; Famvir (TN); Famciclovir [USAN:BAN:INN]; Famciclovir (JAN/USAN/INN); [2-(acetyloxymethyl)-4-(2-aminopurin-9-yl)butyl] acetate; Diacetyl 6-deoxy-9-(4-hydroxy-3-hydroxymethyl-but-1-yl)guanine; 1,3-Propanediol, 2-(2-(2-amino-9H-purin-9-yl)ethyl)-, diacetate (ester); 2-(2-(2-Amino-9H-purin-9-yl)ethyl)-1,3-propanediol diacetate (ester); 2-(2-(2-amino-9H-purin-9-yl)ethyl)-1,3-propanediol diacetate; 2-(acetoxymethyl)-4-(2-amino-4,5-dihydro-9H-purin-9-yl)butyl acetate; 2-[(acetyloxy)methyl]-4-(2-amino-9H-purin-9-yl)butyl acetate; 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate; 9-(4-acetoxy-3-(acetoxymethyl)but-1-yl)-2-aminopurine; 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine
|