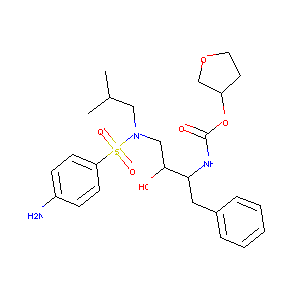
| Synonyms |
AMV; Agenerase; Amprenavir[usan]; Prozei; Vertex; Amprenavir [USAN]; VX 478; VX478; Vertex VX478; Agenerase (TM); Agenerase (TN); DRG-0258; GNA & Amprenavir; HHA & Amprenavir; KVX-478; VX-478; Amprenavir (JAN/USAN/INN); Tetrahydro-3-furyl N-(3-(4-amino-N-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl)carbamate; {3-[(4-AMINO-BENZENESULFONYL)-ISOBUTYL-AMINO]-1-BENZYL-2-HYDROXY-PROPYL}-CARBAMIC ACID TETRAHYDRO-FURAN-3-YL ESTER; [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester & Galanthus nivalis agglutinin (GNA); Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester & Hippeastrum hybrid agglutinin(HHA); (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamate; (3S)-Tetrahydro-3-furyl ((alphaS)-alpha-((1R-1-hydroxy-2-(N(sup 1)-isobutylsulfanilamido)ethyl)phenethyl)carbamate; (3S)-tetrahydro-3-furyl N-[(1S,2R)-3-(4-amino-N-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxy-propyl]carbamate; (3S-(3R*(1R*,2S*)))-(3-(((4-Aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl) tetrahydro-3-furanyl carbamate; 4-Amino-N-((2 syn,3S)-2-hydroxy-4-phenyl-3-((S)-tetrahydrofuran-3-yloxycarbonylamino)-butyl)-N-isobutyl-benzenesulfonamide
|