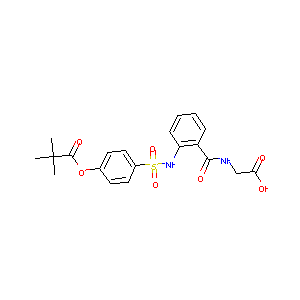
| Synonyms |
sivelestat sodium hydrate; Sivelestat sodium tetrahydrate; 201677-61-4; Elaspol; Sodium sivelestat; EI546 sodium; UNII-737RR8Y409; Sivelestat (sodium tetrahydrate); LY544349 sodium; Sivelestat sodium [USAN]; Sivelestat sodium (USAN); 737RR8Y409; DSSTox_CID_26727; DSSTox_RID_81857; DSSTox_GSID_46727; sodium 2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetate tetrahydrate; LY544349 Sodium Hydrate; Elaspol (TN); CAS-201677-61-4; NCGC00167577-01; AC1L4KMF; Sivelestat sodium hydrate (JAN); Sodium 2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetate tetrahydrate; Sodium ((2-(((4-((2,2-dimethylpropanoyl)oxy)phenyl)sulfonyl)amino)benzoyl)amino)acetate tetrahydrate; Glycine, N-(2-(((4-(2,2-dimethyl-1-oxopropoxy)phenyl)sulfonyl)amino)benzoyl)-, monosodium salt, tetrahydrate
|