Details of the Drug
General Information of Drug (ID: DM032YM)
| Drug Name |
2-pentenal
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
trans-2-Pentenal; 2-PENTENAL; 1576-87-0; (E)-2-Pentenal; (E)-pent-2-enal; 3-Ethylacrolein; 764-39-6; pent-2-enal; 2-Pentenal, (E)-; 2-(E)-Pentenal; 3-Ethyl-2-propenal; (E)-Pent-2-en-1-al; gamma-Methylcrotonaldehyde; 2-Penten-1-al; trans-2-Penten-1-al; UNII-7A4R3CQA2T; FEMA No. 3218; CCRIS 3514; CCRIS 4564; Pentenal, (E)-; 2-Ethylacrylic aldehyde; (E)-2-penten-1-al; EINECS 212-120-2; EINECS 216-414-1; BRN 1719742; 7A4R3CQA2T; CHEMBL256368; DTCCTIQRPGSLPT-ONEGZZNKSA-N; MFCD00009615; trans-2-Pentenal, 97%; Propylidenacetaldehyd
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
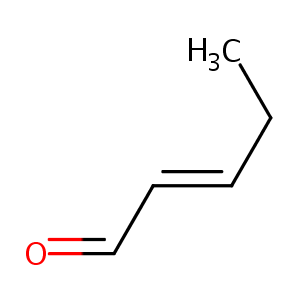 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 84.12 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
