Details of the Drug
General Information of Drug (ID: DM059OH)
| Drug Name |
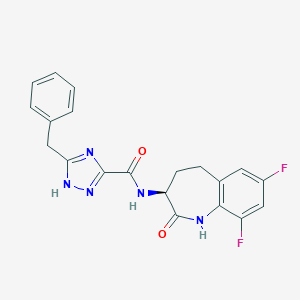
GSK3145095
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1622849-43-7; CHEMBL4452233; (S)-5-benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-yl)-4H-1,2,4-triazole-3-carboxamide; UNII-B4D3WPS7JY; B4D3WPS7JY; SCHEMBL17312826; BCP31015; EX-A3069; BDBM50502339; s8845; GSK-3145095; HY-111946; CS-0094287; GSK-3145095; GSK 3145095; FC1=CC2=C(NC(=O)[C@H](CC2)NC(=O)C2=NN=C(CC3=CC=CC=C3)N2)C(F)=C1; (S)-5-Benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1hbenzo(b)azepin-3-yl)-1H-1,2,4-triazole-3-carboxamide (7,7-dimethyl-2- oxobicyclo(2.2.1)heptan-1-yl)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 397.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
