| Drug Name |
Diaryl amine derivative 1
|
| Synonyms |
PMID26161698-Compound-23 |
| Drug Type |
Small molecular drug
|
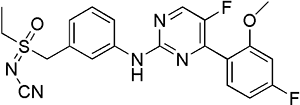
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
443.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.3 |
| Rotatable Bond Count (rotbonds) |
7 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
9 |
| Chemical Identifiers |
- Formula
- C21H19F2N5O2S
- IUPAC Name
[ethyl-[[3-[[5-fluoro-4-(4-fluoro-2-methoxyphenyl)pyrimidin-2-yl]amino]phenyl]methyl]-oxo-lambda6-sulfanylidene]cyanamide - Canonical SMILES
-
CCS(=NC#N)(=O)CC1=CC(=CC=C1)NC2=NC=C(C(=N2)C3=C(C=C(C=C3)F)OC)F
- InChI
-
InChI=1S/C21H19F2N5O2S/c1-3-31(29,26-13-24)12-14-5-4-6-16(9-14)27-21-25-11-18(23)20(28-21)17-8-7-15(22)10-19(17)30-2/h4-11H,3,12H2,1-2H3,(H,25,27,28)
- InChIKey
-
QEYANDLEJXFZFA-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 71515733
- TTD ID
- D0P4NP
|
|
|
|
|
|
|
|


