Details of the Drug
General Information of Drug (ID: DM0A1YI)
| Drug Name |
1na
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3946-01-8; Methyl 2-acetamido-2-deoxy-beta-D-glucopyranoside; N-((2R,3R,4R,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3-yl)acetamide; Alpha-Methyl-N-Acetyl-D-Glucosamine; Beta-Methyl-N-Acetyl-D-Glucosamine; Methyl 2-acetamido-2-deoxy-b-D-glucopyranoside; AC1L9IIR; SCHEMBL1673962; ZEVOCXOZYFLVKN-JGKVKWKGSA-N; MolPort-000-189-911; ZINC5234411; methyl n-acetyl-beta-d-glucosaminide; AKOS002687842; N-ACETYL-O-METHYL-D-GLUCOSAMINE; DB04426; N-acetyl-1-O-methyl-beta-d-glucosamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
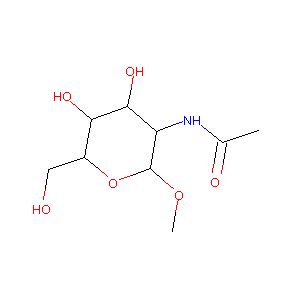 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 235.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
