Details of the Drug
General Information of Drug (ID: DM0BL2G)
| Drug Name |
Mitiperstat
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Mitiperstat; AZD4831; Mitiperstat [INN]; AZD-4831; S6GYK3X4QQ; 1933460-19-5; UNII-S6GYK3X4QQ; AZD-4831 [WHO-DD]; 1-((2-((1R)-1-Aminoethyl)-4-chloro-phenyl)methyl)-2-thioxo-5hpyrrolo(3,2-d)pyrimidin-4-one; 4H-Pyrrolo(3,2-d)pyrimidin-4-one, 1-((2-((1R)-1-aminoethyl)-4-chlorophenyl)methyl)-1,2,3,5-tetrahydro-2-thioxo-; 4H-Pyrrolo[3,2-d]pyrimidin-4-one, 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-1,2,3,5-tetrahydro-2-thioxo-; Alternative Preparation; MITIPERSTAT [USAN]; Azd 4831; CHEMBL5095218; SCHEMBL17782047; GTPL12154; AZD 4831 [WHO-DD]; BHKKSKOHRFHHIN-MRVPVSSYSA-N; BDBM312172; GLXC-26157; EX-A7129; compound 16 [PMID: 36005476]; HY-145581; CS-0376445; US9616063, 3; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1H,2H,3H,4H,5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-{2-[(1R)-1-Aminoethyl]-4-chlorobenzyl}-2-thioxo-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
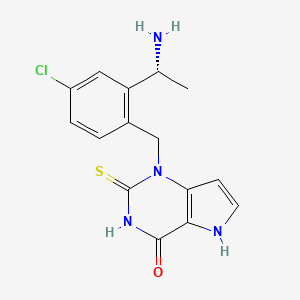 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Heart failure with preserved ejection fraction | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD11.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
