Details of the Drug
General Information of Drug (ID: DM0FGDO)
| Drug Name |
ACCLAIM
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Depon; Excel; Furore; PUMA; Whip; Fenoxaprop ethyl; Fenoxaprop ethyl ester; Fenoxaprop ethyl ester [ANSI]; Tiller Herbicide; HOE 33171; FENOXAPROP-ETHYL; HOE-A 25-01; Ethyl 2-(4-((6-chlorobenzoxazol-2-yl)oxy)phenoxy)propionate; Ethyl 2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoate; Ethyl (D+)-2-(4-(6-chlor-2-benzoxazolyloxy)phenoxy)propanoate; Ethyl (D+)-2-(4-(6-chlor-2-benzoxazolyloxy)phenoxy)propanoate [French]; Ethyl 2-(4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy)propanoate; Ethyl 2-{4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy}propanoate; Ethyl-2-((4-(6-chloro-2-benzoxazolyloxy))-phenoxy)propionate; Ethyl (+-)-2-(4-(6-chloro-2-benzoxazolyloxy)phenoxy)propanoate; Propanoic acid, 2-(4-((6-chloro-2-benzoxazolyl)oxy)phenoxy)-, ethyl ester; (+/-)-2-(4-((6-Chloro-2-benzoxazolyl)oxy)phenoxy)propanoic acid ethyl ester; 2-(4-((6-Chloro-2-benzoxazolylen)oxy)phenoxy)propanoic acid, ethyl ester
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
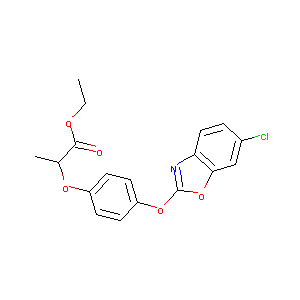 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 361.8 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
