Details of the Drug
General Information of Drug (ID: DM0L6E1)
| Drug Name |
Cycloleucine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
cycloleucine; 1-Aminocyclopentanecarboxylic acid; 52-52-8; 1-Aminocyclopentane-1-carboxylic acid; Cycloleucin; 1-Amino-1-cyclopentanecarboxylic acid; 1-Amino-1-carboxycyclopentane; 1-Amino-cyclopentanecarboxylic acid; CYCLO-LEUCINE; Cyclopentanecarboxylic acid, 1-amino-; NSC 1026; CB 1639; X 201; UNII-0TQU7668EI; 1-Aminocyclopentanecarboxylate; HSDB 5195; WR 14,997; NSC1026; 1-amino cyclopentane carboxylic acid; EINECS 200-144-6; BRN 0636626; aminocyclopentanecarboxylic acid; Cyclopentanecarboxylic acid, 1-amino-, L-; AI3-26442
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
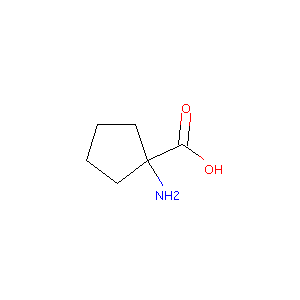 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 129.16 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
