Details of the Drug
General Information of Drug (ID: DM14XB7)
| Drug Name |
PT-317
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
149486-80-6; Thiourea, N-(4-(1-methylethyl)-2-thiazolyl)-N'-(2-phenylethyl)-; PETT Analog 40; Thiourea, N-[4-(1-methylethyl)-2-thiazolyl]-N'-(2-phenylethyl)-; PT-317; PETT i-Pr deriv.; AC1MHDKR; CHEMBL254839; BDBM1873; DTXSID90164307; ZINC13744897; 1-Phenethyl-3-(4-isopropyl-2-thiazolyl)thiourea; 1-(4-isopropylthiazol-2-yl)-3-phenethyl-thiourea; N-(2-Phenethyl)-N -(2-(4-isopropylthiazolyl))thiourea; 1-phenethyl-3-(4-propan-2-yl-1,3-thiazol-2-yl)thiourea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
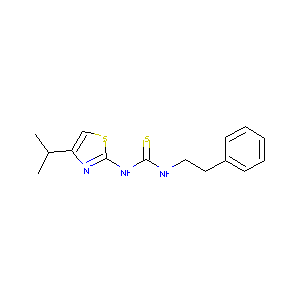 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 305.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
