Details of the Drug
General Information of Drug (ID: DM15YL0)
| Drug Name |
ML204
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5465-86-1; ML204; ML 204; 4-methyl-2-(piperidin-1-yl)quinoline; 4-Methyl-2-(1-piperidinyl)-quinoline; 4-methyl-2-piperidin-1-ylquinoline; 4-Methyl-2-(1-piperidinyl)quinoline; VU0024172-3; NSC25850; AC1Q4W9R; Cambridge id 5563912; AC1L5K4A; MLS001007138; 4-methyl-2-piperidylquinoline; 4-methyl-2-piperidinoquinoline; 2-Piperidino-4-methylquinoline; GTPL4255; cid_230710; 4-methyl-2-piperidino-quinoline; SCHEMBL14975993; CHEMBL1459962; CTK5A2131; BDBM77617; DTXSID30282438; EX-A938; AOB1526; MolPort-000-220-757; HMS2704E20; BCP28617
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
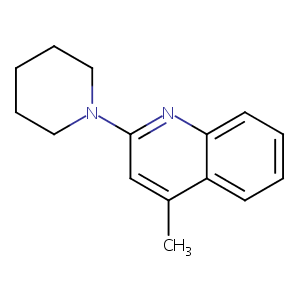 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 226.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
