Details of the Drug
General Information of Drug (ID: DM1DNRP)
| Drug Name |
(S)-5-iodowillardiine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
IODO-WILLARDIINE; S-5-Iodowillardiine; (S)-(-)-5-IODOWILLARDIINE; (s)-5-iodowillardiine; 140187-25-3; CHEMBL121915; 2-AMINO-3-(5-IODO-2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-PROPIONIC ACID; IWD; 5 Iodowillardine; 5-iodowillardiine; 1mqg; Tocris-0307; AC1L9KKQ; Lopac0_001239; SCHEMBL401659; GTPL4071; SCHEMBL13319969; DTXSID90332246; MolPort-006-069-040; ZINC2047688; BDBM50060627; AKOS024457411; DB02818; CCG-205313; NCGC00024529-01; NCGC00024529-02; EU-0101239; B6252; W-110; SR-01000597629; SR-01000075424; SR-01000075424-1; J-007367
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
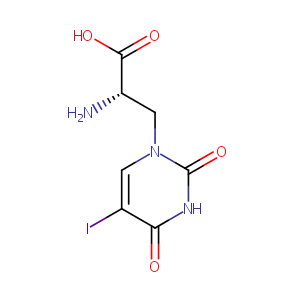 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 325.06 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
