Details of the Drug
General Information of Drug (ID: DM1PRT6)
| Drug Name |
M710
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
637-01-4; N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride; N1,N1,N4,N4-tetramethylbenzene-1,4-diamine dihydrochloride; N,N,N',N'-Tetramethyl-1,4-phenylenediamine Dihydrochloride; UNII-66W8HKA51X; MFCD00012482; 66W8HKA51X; tetramethyl-p-phenylenediamine dihydrochloride; 1,4-Benzenediamine, N,N,N',N'-tetramethyl-, dihydrochloride; NSC36730; 1,4-Benzenediamine, N1,N1,N4,N4-tetramethyl-, hydrochloride (1:2); 1-N,1-N,4-N,4-N-tetramethylbenzene-1,4-diamine;dihydrochloride; Wursters Reagent; N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride, 99%; EINECS 211-274-8; NSC 36730; TMPPD; ACMC-1B8O0; SCHEMBL379125; DTXSID2060915; N,N,N',N'-Tetramethyl-para-phenylenediamine dihydrochloride; ANW-34710; AKOS005254702; MCULE-3840175847; VZ32824; AS-14819; SY061584; 3-(4-Bromo-1H-pyrazol-1-yl)propanoicacid; DB-054526; FT-0629355; ST50308398; Tetramethyl-p-phenylene diamine hydrochloride; 1,4-Bis(dimethylamino)benzene dihydrochloride; X-4255; N,N,N',N'-Tetramethyl-p-phenylenediamine 2HCl; N,N,N',N'-Tetramethyl-p-phenylenediamine DiHCl; W-104885; Q27264036; [4-(dimethylamino)phenyl]dimethylamine, chloride, chloride; N,N,N\\',N\\'-Tetramethyl-p-phenylenediamine dihydrochloride; N1,N1,N4,N4-Tetramethyl-1,4-benzenediamine Dihydrochloride; p-Phenylenediamine, N,N,N',N'-tetramethyl-, dihydrochloride (8CI); N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride, >=95%, powder; N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride, >=97.0% (AT)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
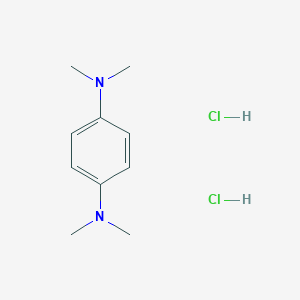 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 237.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
