Details of the Drug
General Information of Drug (ID: DM1PXAU)
| Drug Name |
GS-5894
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2,4-Dichloro-5-iodopyrimidine; 13544-44-0; 2, 4-dichloro-5-iodopyrimidine; 5-iodo-2,4-dichloropyrimidine; MFCD01898087; 2,4-Dichloro-5-iodo-pyrimidine; NSC97872; NCIOpen2_006533; 2,4-Chloro-5-iodopyrimidine; SCHEMBL117265; 5-iodo-2, 4-dichloropyrimidine; DTXSID90294754; RGJNPJRAXMSHKN-UHFFFAOYSA-N; BCP22998; NSC 97872; NSC-97872; AKOS002665245; 2,4-Dichloro-5-iodopyrimidine, 95%; AB10498; AC-1289; CS-W002862; GS-5894; SY009554; AM20080261; D4122; FT-0602319; EN300-252921; W-201138; Z1269214376
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
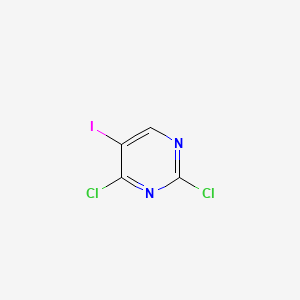 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
