Details of the Drug
General Information of Drug (ID: DM276N1)
| Drug Name |
PIRITREXIM
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Piritrexim; 72732-56-0; Piritrexim [INN]; Piritreximum [Latin]; Piritrexime [French]; 6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine; Piritrexima [Spanish]; BW 301U; UNII-MK2A783ZUT; BW-301U; TCMDC-137235; BRN 5768301; MK2A783ZUT; CHEMBL7492; 2,4-Diamino-5-methyl-6-(2,5-dimethoxybenzyl)pyrido(2,3-d)pyrimidine; 6-(2,5-DIMETHOXY-BENZYL)-5-METHYL-PYRIDO[2,3-D]PYRIMIDINE-2,4-DIAMINE; 6-((2,5-Dimethoxyphenyl)methyl)-5-methylpyrido(2,3-d)pyrimidine-2,4-diamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
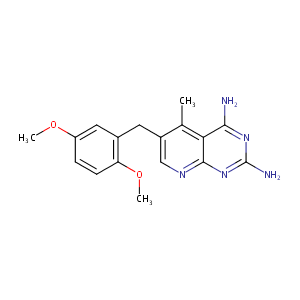 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 325.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
