Details of the Drug
General Information of Drug (ID: DM2AYUX)
| Drug Name |
CC-100
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fendosal; 53597-27-6; 5-(4,5-Dihydro-2-phenyl-3H-benz[e]indol-3-yl)salicylic acid; Alnovin; Fendosalum; HP 129; Fendosalum [INN-Latin]; UNII-9Z709558TZ; P 71-0129; C25H19NO3; NSC 351522; BRN 1665211; 2-hydroxy-5-(2-phenyl-4,5-dihydrobenzo[e]indol-3-yl)benzoic acid; 2-Hydroxy-5-(2-phenyl-4,5-dihydro-3H-benzo[e]indol-3-yl)benzoic acid; 3-(3-Carboxy-4-hydroxyphenyl)-2-phenyl-4,5-dihydro-3H-benz(e)indole; 5-(4,5-Dihydro-2-phenyl-3H-benz(e)indol-3-yl)-2-hydroxybenzoic acid; NSC351522; HP-129; 9Z709558TZ
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
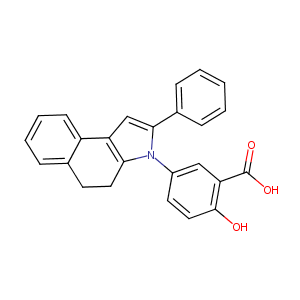 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
