Details of the Drug
General Information of Drug (ID: DM2FJE7)
| Drug Name |
NDI-034858
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zasocitinib; NDI-034858; C293MNS6TQ; 2272904-53-5; UNII-C293MNS6TQ; TAK-279; Pyrazolo(1,5-a)pyrimidine-3-carboxamide, N-((1R,2R)-2-methoxycyclobutyl)-7-(methylamino)-5-((2-oxo(1(2H),2'-bipyridin)-3-yl)amino)-; (8S)-N-[(1R,2R)-2-methoxycyclobutyl]-7-(methylamino)-5-{[(1P,2'P)-2-oxo-2H-[1,2'-bipyridin]-3-yl]amino}pyrazolo[1,5-a]pyrimidine-3-carboxamide; N-((1R,2R)-2-Methoxycyclobutyl)-7-(methylamino)-5-((2-oxo-2H-[1,2'-bipyridin]-3-yl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxamide; zasocitinib [INN]; TAK279; SCHEMBL20678921; GTPL12155; BDBM424361; EX-A7221; NDI034858; US10508120, Compound I-816; US10508120, Compound I-908; MS-28444; HY-150096; CS-0609920; compound I-908 [US20190256519A1]; F96153; N-[(1R,2R)-2-methoxycyclobutyl]-7-(methylamino)-5- [(2-oxo[1(2H),2'-bipyridin]-3-yl)amino]-pyrazolo[1,5- a]pyrimidine-3-carboxamide; N-[(1R,2R)-2-methoxycyclobutyl]-7-(methylamino)-5-[(2-oxo-1-pyridin-2-ylpyridin-3-yl)amino]pyrazolo[1,5-a]pyrimidine-3-carboxamide; ZSB
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
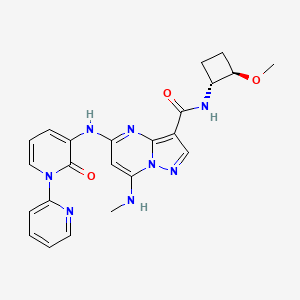 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
