Details of the Drug
General Information of Drug (ID: DM2MYNQ)
| Drug Name |
Piclidenoson
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | IB-MECA; 152918-18-8; piclidenoson; CF-101; 3-IB-Meca; N(6)-Ibamu; CF 101; Cf101; UNII-30679UMI0N; CHEMBL119709; CHEBI:73286; 30679UMI0N; RPR-113090; IB-Meca | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
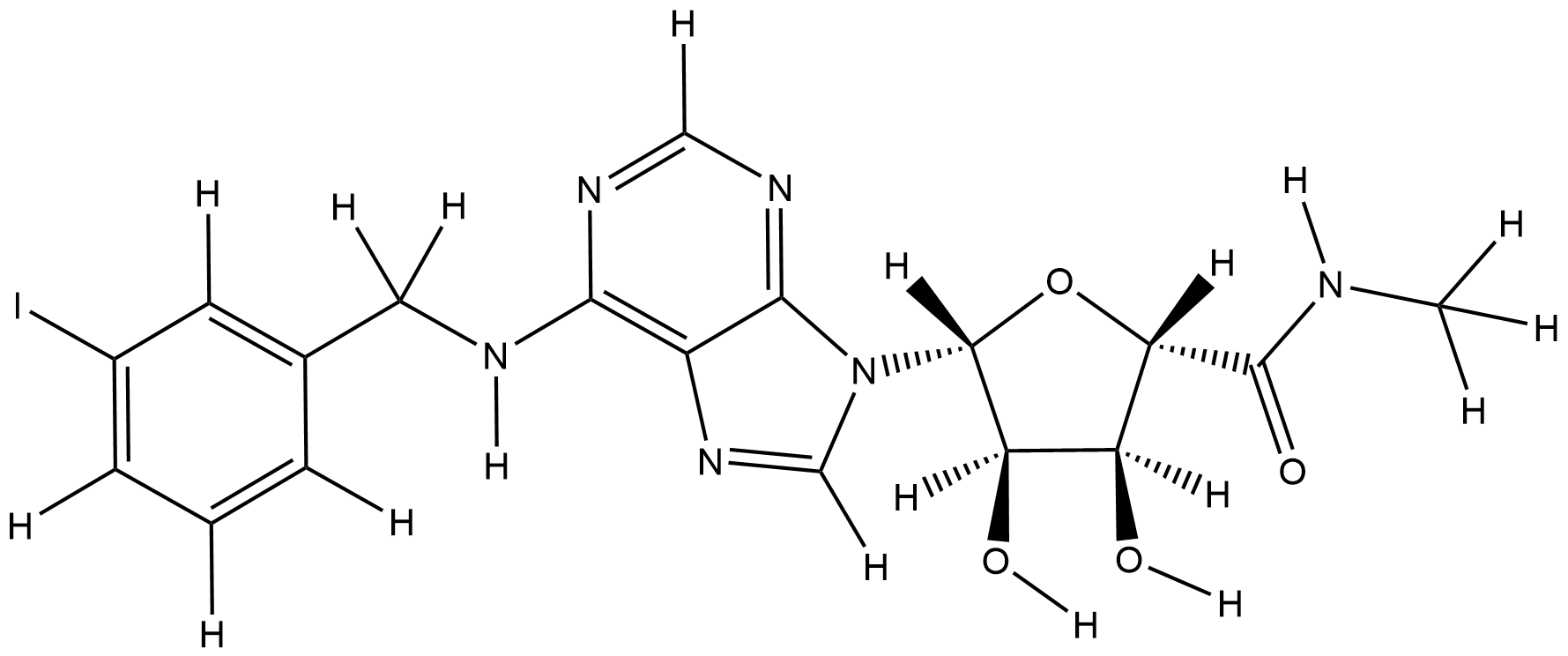 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
