Details of the Drug
General Information of Drug (ID: DM2OHVJ)
| Drug Name |
N-(4-Phenylthiazol-2-yl)isonicotinamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N-(4-phenyl-1,3-thiazol-2-yl)pyridine-4-carboxamide; CHEMBL482012; 5245-66-9; N-(4-Phenylthiazol-2-yl)isonicotinamide; BAS 03572091; AC1Q5OBS; AC1LG7OX; CBMicro_015073; n-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Cambridge id 5245669; Oprea1_303023; Oprea1_553111; SCHEMBL17107144; CTK4J5937; DTXSID30355050; MolPort-001-992-732; ZINC290573; STK483947; BDBM50255300; AKOS000569765; MCULE-9247268363; BIM-0015234.P001; N-(4-Phenyl-thiazol-2-yl)-isonicotinamide; ST50017829; N~4~-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Z27772062
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
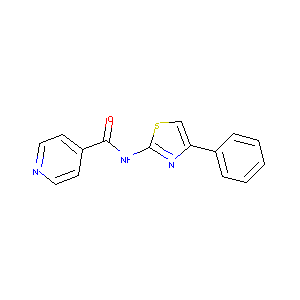 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 281.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
