Details of the Drug
General Information of Drug (ID: DM2S496)
| Drug Name |
Redoxal
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
52962-95-5; MLS000736810; NSC73735; UNII-Q0VK2156M5; N,N'-BIS(o-CARBOXYPHENYL)-o-DIANISIDINE; Q0VK2156M5; SMR000528333; 2,2'-((3,3'-dimethoxy(1,1'-biphenyl)-4,4'-diyl)diimino)bis-benzoic acid; Benzoic acid, 2,2'-((3,3'-dimethoxy(1,1'-biphenyl)-4,4'-diyl)diimino)bis-; 2-[4-[4-(2-carboxyanilino)-3-methoxyphenyl]-2-methoxyanilino]benzoic acid; 2-[4-[4-(2-carboxyanilino)-3-methoxy-phenyl]-2-methoxy-anilino]benzoic acid; 2-[(4-{4-[(2-carboxyphenyl)amino]-3-methoxyphenyl}-2-methoxyphenyl)amino]benzoic acid; Mini-antibody(scFv)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
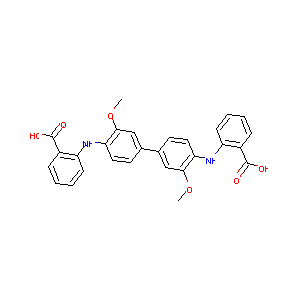 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 484.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
