Details of the Drug
General Information of Drug (ID: DM2U9RY)
| Drug Name |
3-amino-5-(4-chlorophenyl)thiophene-2-carboxamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3-amino-5-(4-chlorophenyl)thiophene-2-carboxamide; 175137-05-0; CHEMBL444884; 515142-45-7; 2-Thiophenecarboxamide,3-amino-5-(4-chlorophenyl)-; AC1MBTGN; 3-amino-5-(4-chlorophenyl)-2-thiophenecarboxamide; SCHEMBL4097325; CTK4D5319; DTXSID90373351; MQYJPACEBWKWEH-UHFFFAOYSA-N; MolPort-000-150-990; ZX-AT016871; ZINC2556119; SBB101177; BDBM50186632; 5704AD; AKOS022181810; AJ-39853; ACM175137050; RT-019539; FT-0704094; 3-Amino-5-(4-chloro-phenyl)-thiophene-2-carboxylic acid amide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
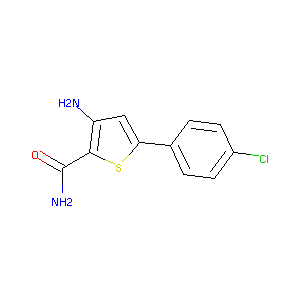 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 252.72 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
