Details of the Drug
General Information of Drug (ID: DM2WZCX)
| Drug Name |
6-Nitroindazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
6-Nitro-1H-indazole; 6-Nitroindazole; 7597-18-4; 6-Nitro-2H-indazole; 6-Nitroisoindazole; 1H-INDAZOLE, 6-NITRO-; 65750-02-9; 2H-Indazole, 6-nitro-; CCRIS 3263; EINECS 231-500-9; NSC 35066; NSC 56816; BRN 0007812; CHEMBL54277; MLS000069593; ORZRMRUXSPNQQL-UHFFFAOYSA-N; SMR000059016; 6NI; 6-nitro-indazol; indazole,6-nitro; Tocris-0710; PubChem20594; Opera_ID_909; 2H-Indazole,6-nitro-; AC1Q1XVW; 6-Nitroindazole, 97%; ACMC-209p0z; AC1L2NB1; AC1Q1Y5L; MLS001148387; KSC379M3P; 5-23-06-00183 (Beilstein Handbook Reference); SCHEMBL271522
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
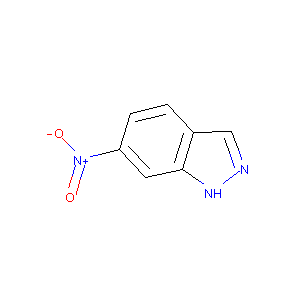 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 163.13 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
