| Drug Name |
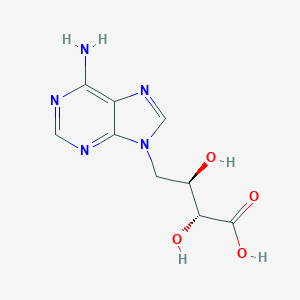
D-Eritadenine
|
| Synonyms |
AC1O59J4; ZINC3872190; (2S,3R)-4-(6-aminopurin-9-yl)-2,3-dihydroxybutanoic acid; (2S,3R)-4-(6-aminopurin-9-yl)-2,3-dihydroxy-butanoic acid; 9H-Purine-9-propanoic acid, 6-amino-alpha-hydroxy-, (alphaR)-
|
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
253.22 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-1.8 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
4 |
| Hydrogen Bond Acceptor Count (hbondacc) |
8 |
| Chemical Identifiers |
- Formula
- C9H11N5O4
- IUPAC Name
(2R,3R)-4-(6-aminopurin-9-yl)-2,3-dihydroxybutanoic acid - Canonical SMILES
-
C1=NC(=C2C(=N1)N(C=N2)C[C@H]([C@H](C(=O)O)O)O)N
- InChI
-
InChI=1S/C9H11N5O4/c10-7-5-8(12-2-11-7)14(3-13-5)1-4(15)6(16)9(17)18/h2-4,6,15-16H,1H2,(H,17,18)(H2,10,11,12)/t4-,6-/m1/s1
- InChIKey
-
LIEMBEWXEZJEEZ-INEUFUBQSA-N
|
| Cross-matching ID |
- PubChem CID
- 159961
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0G3CQ
|
|
|
|
|
|
|
|

