Details of the Drug
General Information of Drug (ID: DM31YGM)
| Drug Name |
Monoctanoin component C
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DICAPRYLIN; MONOCTANOIN COMPONENT C; 1069-87-0; 3-Hydroxypropane-1,2-diyl dioctanoate; 36354-80-0; diC8; Octanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester; 1,2-Dicapryloylglycerol; 1,2-Dioctanoyl-rac-glycerol; 1,2-Dioctanoin; Octanoin, 1,2-di-; Glycerol 1,2-dicaprylate; AC1Q5YA4; SCHEMBL825310; CHEMBL24609; AC1L1B82; CTK4A4899; ZQBULZYTDGUSSK-UHFFFAOYSA-N; HSCI1_000084; IN1353; AKOS027383004; ACM36354800; (3-hydroxy-2-octanoyloxypropyl) octanoate; FT-0748382; 1-hydroxy-3-(octanoyloxy)propan-2-yl octa
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
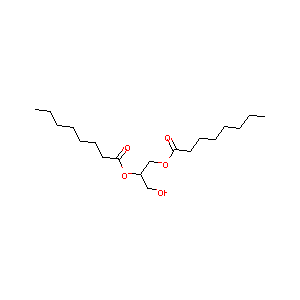 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 344.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 18 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
