Details of the Drug
General Information of Drug (ID: DM3BS97)
| Drug Name |
Macrolides
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tylosin; tylosin tartrate; Tilosina; Tylosinum; UNII-YEF4JXN031; Tylosine; Tylocine; Tylan; Tylosin A; 1401-69-0; YEF4JXN031; Fradizine; CHEBI:17658; Vubityl 200; Tylosinum [INN-Latin]; Tylosine [INN-French]; Tilosina [INN-Spanish]; HSDB 7022; EINECS 215-754-8; AI3-29799; SR-05000002057; Tylosin [USP:INN:BAN]; Tylan (TN); Tylosin (USP/INN); AC1NQX0W
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
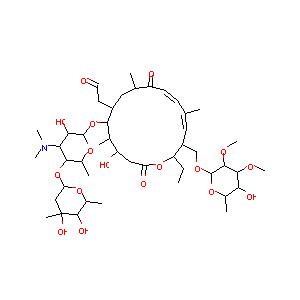 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 916.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 18 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
