Details of the Drug
General Information of Drug (ID: DM3C8GQ)
| Drug Name |
PF-489791
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-2S27T3DSZ3; PF-489791; PF-00489791; 853003-48-2; 2S27T3DSZ3; PF-489,791; ZUHZNKJIJDAJFD-UHFFFAOYSA-N; UK-489,791; SCHEMBL331279; SCHEMBL8042129; GTPL8377; DB11736; SB16722; 1H-Pyrazolo(4,3-d)pyrimidine-3-carboxamide, 1-(2-ethoxyethyl)-5-(ethylmethylamino)-7-((4-methyl-2-pyridinyl)amino)-N-(methylsulfonyl); n-[1-(2-ethoxyethyl)-5-(n-ethyl-n-methylamino)-7-(4-methylpyridin-2-yl-amino)-1h-pyrazolo[4,3-d]pyrimidine-3-carbonyl]methanesulfonamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
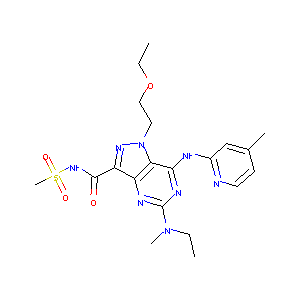 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 476.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic obstructive pulmonary disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA22 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
