Details of the Drug
General Information of Drug (ID: DM3MUF1)
| Drug Name |
4-amino-2H-chromen-2-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Amino-chromen-2-one; 4-Aminocoumarin; 53348-92-8; 4-amino-2H-chromen-2-one; 4-Amino-2H-1-benzopyran-2-one; 2H-1-Benzopyran-2-one, 4-amino-; UNII-SCI2054E4F; CHEMBL240482; SCI2054E4F; Coumarin, 4-amino-; 4-aminochromen-2-one; AC1LGGAS; Coumarin, 4-amino- (7CI); SCHEMBL4396062; AC1Q69X0; CTK7H2649; DTXSID90201509; MolPort-000-000-981; AHZAKFLOHIRCDU-UHFFFAOYSA-N; ZINC337346; STL371135; BDBM50226833; 4-[Amino]-2H-1-benzopyran-2-one; AKOS004909244; FCH1162846; MCULE-3726196442; MB00371; LS-39436; DB-071668; KB-189290; FT-0740005; A58124
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
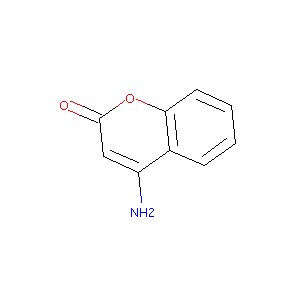 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 161.16 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
