| Drug Name |
M2698
|
| Synonyms |
HXAUJHZZPCBFPN-QGZVFWFLSA-N; 1379545-95-5; SCHEMBL15262358; EX-A1187; AKOS030627134; M2698(MSC-2363318A) |
| Indication |
| Disease Entry |
ICD 11 |
Status |
REF |
| Solid tumour/cancer |
2A00-2F9Z
|
Phase 1 |
[1] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
| Structure |
|
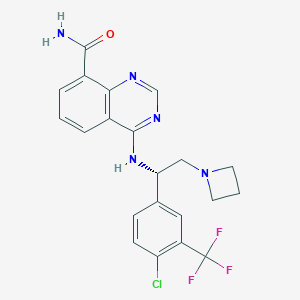 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
449.9 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.9 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
8 |
| Chemical Identifiers |
- Formula
- C21H19ClF3N5O
- IUPAC Name
4-[[(1S)-2-(azetidin-1-yl)-1-[4-chloro-3-(trifluoromethyl)phenyl]ethyl]amino]quinazoline-8-carboxamide - Canonical SMILES
-
C1CN(C1)C[C@H](C2=CC(=C(C=C2)Cl)C(F)(F)F)NC3=NC=NC4=C3C=CC=C4C(=O)N
- InChI
-
InChI=1S/C21H19ClF3N5O/c22-16-6-5-12(9-15(16)21(23,24)25)17(10-30-7-2-8-30)29-20-14-4-1-3-13(19(26)31)18(14)27-11-28-20/h1,3-6,9,11,17H,2,7-8,10H2,(H2,26,31)(H,27,28,29)/t17-/m1/s1
- InChIKey
-
HXAUJHZZPCBFPN-QGZVFWFLSA-N
|
| Cross-matching ID |
- PubChem CID
- 89808643
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D06HYF
|
| Combinatorial Drugs (CBD) |
Click to Jump to the Detailed CBD Information of This Drug
|
|
|
|
|
|
|
|


