Details of the Drug
General Information of Drug (ID: DM3XX3F)
| Drug Name |
Celivarone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Celivarone; UNII-K45001587E; 401925-43-7; K45001587E; Celivarone [INN]; SSR 149744; SCHEMBL3251183; CHEMBL3707403; SSR-149744C; SB17436; SSR-149744; 5-Benzofurancarboxylic acid, 2-butyl-3-(4-(3-(dibutylamino)propyl)benzoyl)-, 1-methylethyl ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
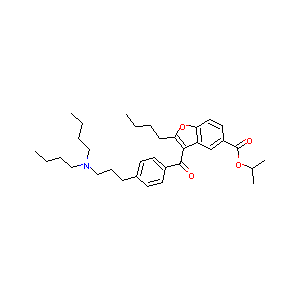 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
References
