Details of the Drug
General Information of Drug (ID: DM49WQQ)
| Drug Name |
Reltecimod
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-U00J02UY29; 1447799-33-8; AB103; U00J02UY29; 1447799-33-8 (free); (S)-3-((S)-2-((S)-2-((S)-2-((S)-2-((S)-2-((S)-1-(D-alanyl-L-seryl)pyrrolidine-2-carboxamido)-4-(methylthio)butanamido)-4-methylpentanamido)-3-methylbutanamido)propanamido)-3-(4-hydroxyphenyl)propanamido)-4-(((R)-1-carboxyethyl)amino)-4-oxobutanoic acid; Reltecimod [INN]; Reltecimod [USAN]; Reltecimod (USAN/INN); Reltecimod [USAN:INN]; CHEMBL3989950; D11281; Q27290502; (5S,8S,11S,14S,17S,20S,23R)-5-((S)-1-((S)-2-((R)-2-Aminopropanamido)-3-hydroxypropanoyl)pyrrolidine-2-carboxamido)-20-(carboxymethyl)-17-(4-hydroxybenzyl)-8-isobutyl-11-isopropyl-14,23-dimethyl-6,9,12,15,18,21-hexaoxo-2-thia-7,10,13,16,19,22-hexaazatetracosan-24-oic acid; D-Alanine, D-alanyl-L-seryl-L-prolyl-L-methionyl-L-leucyl-L-valyl-L-alanyl-L-tyrosyl-L-alpha-aspartyl-; D-Alanyl-(t-cell-specific surface glycoprotein CD28-(8-15)- peptide)-D-alanine:D-alanyl-L-seryl-L-prolyl-L-methionyl-L-leucyl-L-valyl-L-alanyl-L-tyrosyl-L-alpha-aspartyl-D-alanine immunomodulator
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Peptide
|
||||||||||||||||||||||
| Structure |
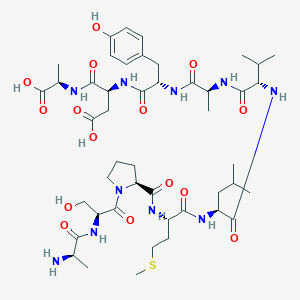 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
