| Drug Name |
Cysteine Sulfenic Acid
|
| Synonyms |
S-Hydroxycysteine; s-hydroxy-l-cysteine; Cysteinesulfenic acid; Cys-sulfenic acid; L-cysteinesulfenic acid; UNII-FB8KIA847T; L-Cysteinsulfensaeure; Alanine, 3-sulfeno-; Cys(OH); FB8KIA847T; L-2-amino-3-sulfeno-propionic acid; 5722-80-5; 2-amino-3-hydroxysulfanylpropionic acid; Cysteine-sulfenic acid; L-Alanine, 3-sulfeno-; S-(Propylcarbamoyl)cysteine; AC1Q5QNT; AC1L4WIO; SCHEMBL333356; CHEBI:41710; CTK1H0247; AKOS006339917; DB01915; L-Cysteine, S-[(propylamino)carbonyl]-; 73243-12-6
|
| Drug Type |
Small molecular drug
|
| Structure |
|
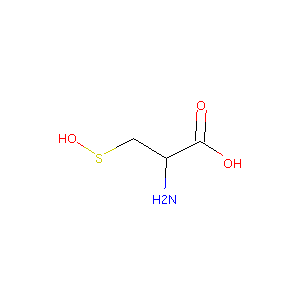 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
137.16 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-3.6 |
| Rotatable Bond Count (rotbonds) |
3 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
5 |
| Chemical Identifiers |
- Formula
- C3H7NO3S
- IUPAC Name
(2R)-2-amino-3-hydroxysulfanylpropanoic acid - Canonical SMILES
-
C([C@@H](C(=O)O)N)SO
- InChI
-
InChI=1S/C3H7NO3S/c4-2(1-8-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1
- InChIKey
-
FXIRVRPOOYSARH-REOHCLBHSA-N
|
| Cross-matching ID |
- PubChem CID
- 165339
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0V6UC
|
|
|
|
|
|
|
|


