| Synonyms |
CP 96345; CP-96345; 132746-60-2; UNII-W22ILA2I52; CP 96344; CP 96,345; CHEMBL16192; W22ILA2I52; 2-(Diphenylmethyl)-N-((2-methoxyphenyl)methyl)-1-azabicyclo(2.2.2)octan-3-amine; CP-96,345; (2S,3S)-cis-2-(Diphenylmethyl)-N-[(2-methoxyphenyl)methyl]-1-azabicyclo[2.2.2]octan-3-amine; (2S,3S)-2-benzhydryl-N-[(2-methoxyphenyl)methyl]quinuclidin-3-amine; (2S,3S)-N-(2-methoxyphenyl)methyl-2-diphenylmethyl-1-azabicyclo[2.2.2]octan-3-amine; 1-Azabicyclo(2.2.2)octan-3-amine, 2-(diphenylmethyl)-N-((2-methoxyphenyl)methyl)-, (2S-cis
|
| Chemical Identifiers |
- Formula
- C28H32N2O
- IUPAC Name
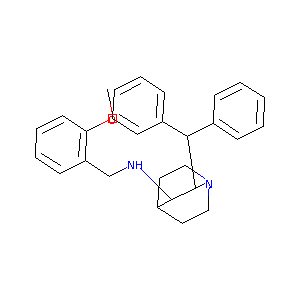
(2S,3S)-2-benzhydryl-N-[(2-methoxyphenyl)methyl]-1-azabicyclo[2.2.2]octan-3-amine - Canonical SMILES
-
COC1=CC=CC=C1CN[C@@H]2[C@@H](N3CCC2CC3)C(C4=CC=CC=C4)C5=CC=CC=C5
- InChI
-
InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1
- InChIKey
-
FLNYLINBEZROPL-NSOVKSMOSA-N
|