Details of the Drug
General Information of Drug (ID: DM4QHZS)
| Drug Name |
BMS-986177
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Milvexian; UNII-0W79NDQ608; Milvexian (USAN); Milvexian [USAN]; BMS 986177; JNJ-70033093; 1802425-99-5; CHEMBL4112929; SCHEMBL16982989; WHO 11401; D11802; (5R,9S)-9-(4-(5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxopyrimidin-1(6H)-yl)-21-(difluoromethyl)-5-methyl-21H-3-aza-1(4,2)-pyridina-2(5,4)-pyrazolacyclonaphan-4-one; (9R,13S)-13-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6-oxo-1,6-dihydropyrimidin-1-yl}-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.02,6]octadeca-1(18),2(6),4,14,16-pentaen-8-one; 11,15-Metheno-15H-pyrazolo(4,3-b)(1,7)diazacyclotetradecin-5(6H)-one, 10-(4-(5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxo-1(6H)-pyrimidinyl)-1-(difluoromethyl)-1,4,7,8,9,10-hexahydro-6-methyl-, (6R,10S)-2
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
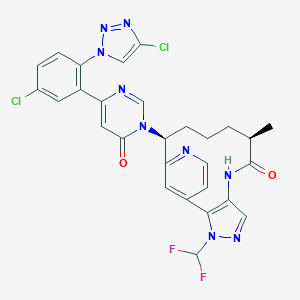 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 626.4 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cerebral ischemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8B11 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
