Details of the Drug
General Information of Drug (ID: DM57TY3)
| Drug Name |
9-ING-41
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1034895-42-5; ND1SOF0DLU; UNII-ND1SOF0DLU; CHEMBL483465; 3-(5-fluoro-1-benzofuran-3-yl)-4-(5-methyl-[1,3]dioxolo[4,5-f]indol-7-yl)pyrrole-2,5-dione; 3-(5-fluorobenzofuran-3-yl)-4-(5-methyl-5H-[1,3]dioxolo[4,5-f]indol-7-yl)-1H-pyrrole-2,5-dione; elraglusib; SCHEMBL3152351; GTPL11412; EX-A4074; BDBM50267716; s9602; SB19735; compound 26 [PMID: 19338355]; HY-113914; CS-0063319; 1H-Pyrrole-2,5-dione, 3-(5-fluoro-3-benzofuranyl)-4-(5-methyl-5H-1,3-dioxolo(4,5-F)indol-7-yl)-; 3-(5-Fluoro-benzofuran-3-yl)-4-(5-methyl-5H-(1,3)dioxolo(4,5-F)indol-7-yl)-pyrrole-2,5-dione; 3-(5-Fluorobenzofuran-3-yl)-4-(5-methyl-5H-[1,3]dioxolo[4,5-f]indol-7-yl)pyrrole-2,5-dione; 4-(5-methyl-5H-[1,3]dioxolo[4,5-f]-indol-7-yl)-3-(5-fluoro-1-benzofuran-3-yl)-1 h-pyrrole-2,5-dione
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
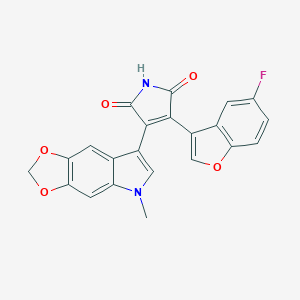 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 404.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Myelofibrosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A20.2 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
