Details of the Drug
General Information of Drug (ID: DM5KSCI)
| Drug Name |
ZD-7155
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ZD-7155; CHEMBL159096; 5,7-diethyl-1-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-3,4-dihydro-1,6-naphthyridin-2-one; 5,7-Diethyl-1-{[2'-(1h-Tetrazol-5-Yl)biphenyl-4-Yl]methyl}-3,4-Dihydro-1,6-Naphthyridin-2(1h)-One; ZD 7155; Tocris-1211; NCGC00025043-01; AC1NGE69; SCHEMBL7286312; GTPL8324; CHEBI:92194; ZINC3784334; BDBM50049187; L007754; BRD-K11373525-003-01-3; ZD7; 5,7-diethyl-1-[2'-(1h-1,2,3,4-tetrazol-5-yl)-bi-phenyl-4-ylmethyl]-1,2,3,4-tetrahydro-1,6-naphthyridin-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
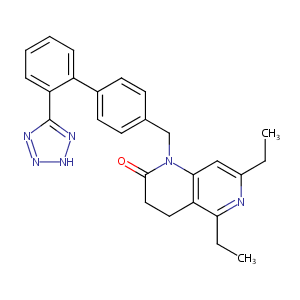 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 438.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
