Details of the Drug
General Information of Drug (ID: DM5OGK0)
| Drug Name |
Epalrestat
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
epalrestat; 82159-09-9; Kinedak; Epalrestatum; Ono 2235; Ono-2235; Epalrestat [INN]; Epalrestatum [Latin]; ONO-2; 2-((z)-5-((e)-2-methyl-3-phenylallylidene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid; ONO 2; UNII-424DV0807X; C15H13NO3S2; CHEMBL56337; 5-((1Z,2E)-2-Methyl-3-phenylpropenylidene)-4-oxo-2-thioxo-3-thiazolidineacetic acid; CHEBI:31539; 5-((Z,E)-beta-Methylcinnamylidene)-4-oxo-2-thioxo-3-thiazolidineacetic acid; 424DV0807X; Epalrestatum; Aldorin (TN); Kinedak (TN); Epalrestat (JAN/INN); {5-[(E)-2-Methyl-3-phenyl-prop-2-en-(Z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl}-acetic acid; {(5Z)-5-[(2E)-2-methyl-3-phenylprop-2-en-1-ylidene]-4-oxo-2-thioxo-1,3-thiazolidin-3-yl}acetic acid; 2-[(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]acetic acid; 3-carboxymethyl-5-(methyl-3-phenylpropenylidene)rhodanine; Ono 2
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
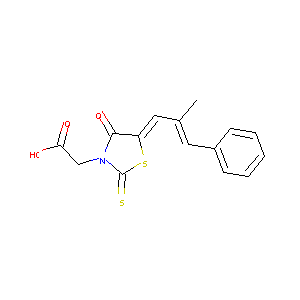 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 319.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Diabetic neuropathy | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8C0Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
