Details of the Drug
General Information of Drug (ID: DM5PFIJ)
| Drug Name |
Givosiran
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1639325-43-1; N-[1,3-Bis[3-[3-[5-[(2R,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxypentanoylamino]propylamino]-3-oxopropoxy]-2-[[3-[3-[5-[(2R,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxypentanoylamino]propylamino]-3-oxopropoxy]methyl]propan-2-yl]-12-[(2R,4R)-4-hydroxy-2-methylpyrrolidin-1-yl]-12-oxododecanamide; Fitusiran; Givlaari; Givosiran [INN]; Givosiran [USAN]; Givosiran [USAN:INN]; ALN-AS1; UNII-ROV204583W; ROV204583W; WHO 10280
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small interfering RNA
|
||||||||||||||||||||||
| Sequence |
>Subunit 1
CAGAAAGAGUGUCUCAUCUUA >Subunit 2 UAAGAUGAGACACUCUUUCUGGU |
||||||||||||||||||||||
| Structure |
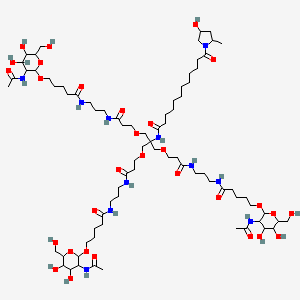 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1711 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -5.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 63 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 20 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 30 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
