Details of the Drug
General Information of Drug (ID: DM5R8KU)
| Drug Name |
1-(4-Methoxy-phenyl)-2-phenyl-ethane-1,2-dione
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Methoxybenzil; p-Methoxybenzil; BENZIL, 4-METHOXY-; 22711-21-3; Ethanedione, (4-methoxyphenyl)phenyl-; NSC 39465; 1-(4-methoxyphenyl)-2-phenylethane-1,2-dione; BRN 2052507; NTINAJCDYRYMML-UHFFFAOYSA-N; NSC39465; Dibenzoyl, 4-methoxy; AC1L1LPW; Benzil-based compound, 20; 4-08-00-02532 (Beilstein Handbook Reference); SCHEMBL2156563; CHEMBL192474; BDBM22742; DTXSID40177245; MolPort-001-788-309; 4-Methoxybibenzyl-alpha,beta-dione; ZINC1671392; STK863335; NSC-39465; BBL023172; NSC602911; AKOS000298817; NSC-602911; MCULE-6173981634
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
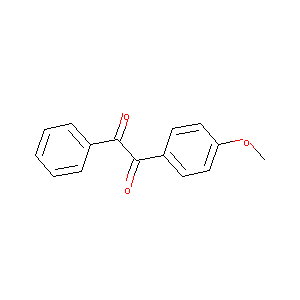 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 240.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
