Details of the Drug
General Information of Drug (ID: DM64CAS)
| Drug Name |
Didox
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
69839-83-4; N,3,4-Trihydroxybenzamide; 3,4-Dihydroxybenzohydroxamic acid; NSC-324360; Benzamide, N,3,4-trihydroxy-; 3,4-Dihydroxyphenylhydroxamic acid; UNII-L106XFV0RQ; VF 147; NSC 324360; DIDO; CCRIS 7909; BRN 2096682; L106XFV0RQ; C7H7NO4; NSC324360; AC1L1F1T; Benzamide,3,4-trihydroxy-; AC1Q5DI9; N-3,4-Tridhydroxybenzamide; SCHEMBL171446; CHEMBL367788; SCHEMBL18732059; Didox, > SCHEMBL18346609; CTK8F9165; DTXSID90220134; QJMCKEPOKRERLN-UHFFFAOYSA-N; MolPort-009-019-216; KS-00001DF2; BCP16846; ZINC3872288; 1716AH
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
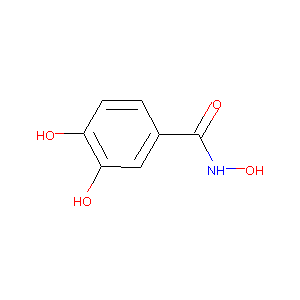 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 169.13 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
