Details of the Drug
General Information of Drug (ID: DM671NG)
| Drug Name |
Progabide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Gabren; Gabrene; Halogabide; Progabida; Progabidum; SL 76002; Gabren (TN); Gabrene (TN); Progabida [INN-Spanish]; Progabidum [INN-Latin]; SL 76-002; SL-76002; Progabide (USAN/INN); Progabide [USAN:BAN:INN]; Progabide [USAN:INN:BAN]; 4-(((4-Chlorophenyl)(5-fluoro-2-hydroxyphenyl)methylene)amino)butanamide; 4-((alpha-(p-Chlorophenyl)-5-fluorosalicylidene)amino)butyramide; 4-(alpha-(p-Chlorophenyl)-5-fluoro-2-hydroxybenzylideneamino)butyramide; 4-[[(4-chlorophenyl)-(3-fluoro-6-oxocyclohexa-2,4-dien-1-ylidene)methyl]amino]butanamide; 4-[[(E)-(4-chlorophenyl)-(3-fluoro-6-oxocyclohexa-2,4-dien-1-ylidene)methyl]amino]butanamide; 4-[[(Z)-(4-chlorophenyl)-(3-fluoro-6-oxocyclohexa-2,4-dien-1-ylidene)methyl]amino]butanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
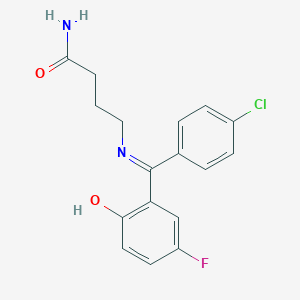 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 334.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
