Details of the Drug
General Information of Drug (ID: DM6KOBZ)
| Drug Name |
TAK-285
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TAK-285; 871026-44-7; TAK 285; TAK285; UNII-70CCB438L6; N-(2-(4-(3-CHLORO-4-(3-(TRIFLUOROMETHYL)PHENOXY)PHENYLAMINO)-5H-PYRROLO[3,2-D]PYRIMIDIN-5-YL)ETHYL)-3-HYDROXY-3-METHYLBUTANAMIDE; CHEMBL1614725; 70CCB438L6; N-{2-[4-({3-Chloro-4-[3-(Trifluoromethyl)phenoxy]phenyl}amino)-5h-Pyrrolo[3,2-D]pyrimidin-5-Yl]ethyl}-3-Hydroxy-3-Methylbutanamide; n-(2-(4-((3-chloro-4-(3-(trifluoromethyl)phenoxy)phenyl)amino)-5h-pyrrolo(3,2-d)pyrimidin-5-yl)ethyl)-3-hydroxy-3-methylbutanamide; C26H25ClF3N5O3
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
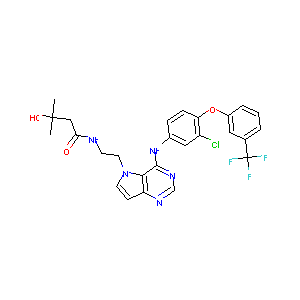 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 548 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
