Details of the Drug
General Information of Drug (ID: DM6L7CL)
| Drug Name |
Symbicort
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(22R)-Budesonide; UNII-2HI1006KPH; 51333-22-3; 51372-29-3; 2HI1006KPH; DSSTox_CID_202; DSSTox_RID_75430; DSSTox_GSID_20202; (1~{s},2~{s},4~{r},6~{r},8~{s},9~{s},11~{s},12~{s},13~{r})-9,13-Dimethyl-11-Oxidanyl-8-(2-Oxidanylethanoyl)-6-Propyl-5,7-Dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icosa-14,17-Dien-16-One; R-Budesonide; Budesonide-22R; NCGC00016862-01; EINECS 257-161-7; CAS-51333-22-3; BUDESONIDE (11beta,16alpha(R)); SCHEMBL4095; AC1L22VC; CHEMBL2110662
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
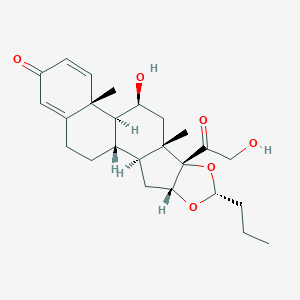 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
