Details of the Drug
General Information of Drug (ID: DM6NPUM)
| Drug Name |
CALCEOLARIOSIDE A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Calceolarioside A; CHEMBL481635; 84744-28-5; AC1NRV65; MEGxp0_000507; ACon1_000369; MolPort-001-740-672; ZINC31156700; BDBM50259819; MCULE-7153533238; NCGC00169142-01; Calceolarioside A, > [(2R,3S,4R,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-4,5-dihydroxy-2-(hydroxymethyl)tetrahydropyran-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate; beta-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl, 4-(3-(3,4-dihydroxyphenyl)-2-propenoate), (E)-; beta-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl 4-O-[(2E)-3-(3
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
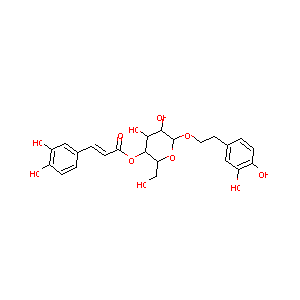 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 478.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
