| Drug Name |
L-NIL
|
| Synonyms |
L-NIL; N(6)-acetimidoyllysine; H-Lys(acetimidoyl)-OH; N~6~-[(1z)-Ethanimidoyl]-L-Lysine; N(6)-acetimidoyl-L-lysine; N(6)-ethanimidoyl-L-lysine; CHEMBL7889; L-N(6)-(1-iminoethyl)lysine; CHEBI:63971; L-N(omega)-(1-iminoethyl)lysine; 53774-63-3; N-(iminoethyl)-L-lysine; L-NIL;H-Lys(1-iminoethyl)-OH;N-(5-Amino-5-carboxypentyl)-acetamidine; L-N6-(1-Iminoethyl)lysine; Tocris-1139; NCGC00015566-01; N6-ethanimidoyl-L-lysine; Lopac-I-8021; AC1MBZ26; (L-N6-1-iminoethyl)lysine; SCHEMBL322091; L-Lysine,n6-(1-iminoethyl)-; DTXSID9041071
|
| Drug Type |
Small molecular drug
|
| Structure |
|
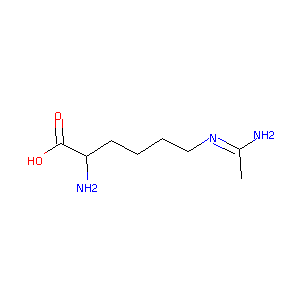 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
187.24 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-3.1 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
- Formula
- C8H17N3O2
- IUPAC Name
(2S)-2-amino-6-(1-aminoethylideneamino)hexanoic acid - Canonical SMILES
-
CC(=NCCCC[C@@H](C(=O)O)N)N
- InChI
-
InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1
- InChIKey
-
ONYFNWIHJBLQKE-ZETCQYMHSA-N
|
| Cross-matching ID |
- PubChem CID
- 2733506
- ChEBI ID
-
- TTD ID
- D0Y7VJ
|
|
|
|
|
|
|
|


