Details of the Drug
General Information of Drug (ID: DM75EAT)
| Drug Name |
(4-Fluoro-phenyl)-(9H-purin-6-yl)-amine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
73663-95-3; N-(p-Fluorophenyl)adenine; (4-Fluorophenyl)(9H-purin-6-yl)amine; NSC 21554; N-(4-fluorophenyl)-9H-purin-6-amine; 7H-ADENINE, N-(p-FLUOROPHENYL)-; N-(4-fluorophenyl)-7H-purin-6-amine; 1H-Purin-6-amine, N-(4-fluorophenyl)-; CHEMBL361227; N-(4-Fluorophenyl)-1H-purin-6-amine; W-203680; (4-Fluoro-phenyl)-(9H-purin-6-yl)-amine; AC1L1C3G; 6-(4-Fluoroanilino)purine; Oprea1_396984; SCHEMBL4922514; N-(p-Fluorophenyl)-1H-adenine; CTK5D8432; (4-fluorophenyl)purin-6-ylamine; DTXSID30223881; MolPort-000-384-310; NSC21554
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
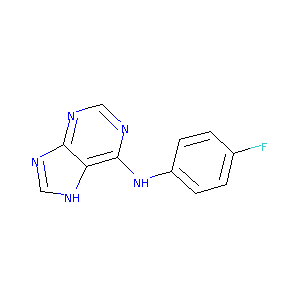 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 229.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
